Estimating Delta G at a given temperature?
In class a few days we are given this problem. I understand the first part because, but I have no idea how to estimate Delta G at 400 K. The answer is provided, but can anyone help inform me on how that answer was obtained?
Use a table of thermodynamic properties to determine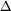
( Read more... )
Use a table of thermodynamic properties to determine
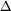
( Read more... )
Comments 2
Longer answer: The temperature-dependent part of ΔG is the change in entropy ΔS multiplied by the temperature. You've just worked out the ΔG and the ΔS for a given T, so from those you just need to work out the other term, the change in enthalpy ΔH. From there you can work out the Gibb's free energy at any temperature just by plugging the values back in to the equation.
ΔG = ΔH - TΔS
Reply
Reply
Leave a comment