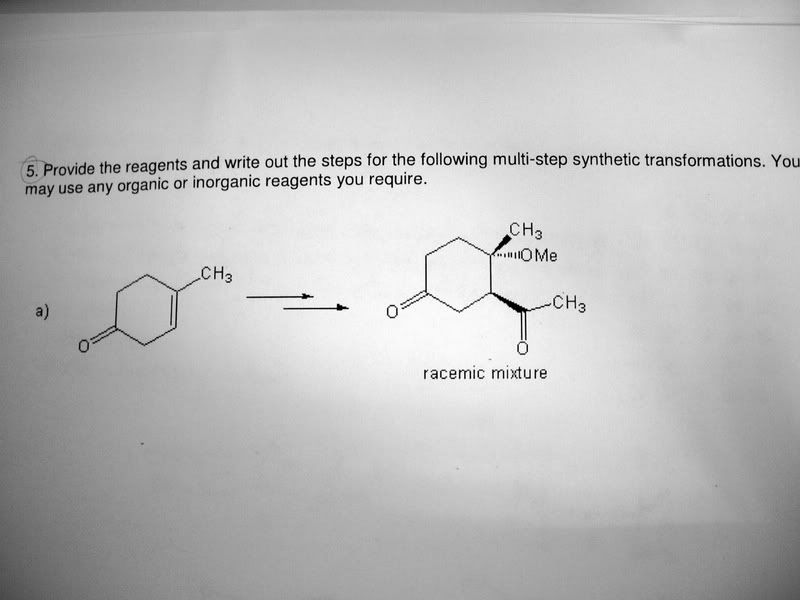
-
Need chem help?
Check out my journal for chemistry help- and more help to come. =] -
Kinetics and Equilibrium powerpoint
Hey, for my HS chemistry class we have to do powerpoints to review for our final. I was wondering if anyone would take a look at my powerpoint and tell me if there's any incorrect information, ways to improve it, and so on? Thank you very much. You can download it hereAlso, if you could tell me if there's any way to make the second half less... ( Read more... ) -
Naming Question
The other day we learned about naming hydrates, as in:
CuSO4 · 5H2O = copper(II) sulfate pentahydrate
My question is how would you name non-hydrate compounds in the same form, like:
Glucosamine sulfate · 2KCl = glucosamine sulfate dipotassium chloride?
Also, what exactly does this mean? My prof said that hydrates are solids that have water in their ( Read more... ) -
Nomenclature Q
Last week we did a lab in gem chem with the following reaction:
NaOH + HC2H3O2 → NaC2H3O2 + H2O.
If I'm not mistaken, HC2H3O2 is acetic acid and C2H3O2 is acetate (the H+ would have been "donated" in the reaction). Would it be correct to call NaC2H3O2 sodium acetate? Could it be called a sodium salt of acetate?
Just curious. Thanks. -
Stupid Stoichiometry
OK I know this is super basic but I don't know how to do this problem: What is the mass, in grams, of one coppur atom?
I thought that mass in grams was equal to amu, but I got that wrong on the test (I put 63.55 g because that was what I had for amu on my periodic table). What to do? -
Chemistry
what's a good way to memorize solubility rules and charges for polyatomic ions? -
weird word/number
ok guys, a friend of mine gave me this problem and between three people, we can not figure it out for the life of us.
you have five single digit numbers represented by a letter S E A M and T. None of these letters are 0, none of them repeat (like M cannot equal S and so on), and T cannot be 1.
so the number made up of SEAM (in that order) ( Read more... ) -
Better question
I am completely stuck, I really could use the help, dealing with coordination compounds which I have no idea how to do.
( My problem ) -
(Untitled)
Concerning Lewis structures, I know that a molecule with polar bonds can be nonpolar if the bond negativities cancel or are symmetrical so that they cancel out, etc., but are double bonds treated the same way? As in the case of BCl3, the lewis structure has the B double-bonded to ONE of the Cl's, and the rest are single bonds, and my question is, ( Read more... )