-
Telecommunications Equipment Markets In China
Aarkstore Enterprise announced latest Market Research Report Titled "Telecommunications Equipment Markets In China"
Chinas demand for Telecommunications Equipment has grown at a fast pace in the past decade. In the next decade, both production and demand will continue to grow. The Chinese economy maintains a high speed growth which has been ( Read more... ) -
Стиральный порошок
после стирки в машине с "обычным порошком" одежда жутко воняет ароматизатором из этого порошка ( Read more... ) -
Oppolzer's Camphorsultam and Arndt-Eistert
Hello everyone!
My supervisor has given me a reaction from before I started my PhD to talk through during a presentation and I'm considering questions that may be asked in the Q&A. I'm struggling to work out how Oppolzer's camphor sultam works. I found one explanation that said "steric hindrance of the dimethyl group in conjunction with repulsion of ( Read more... ) -
Exam Preparation
Hi,
I have a Chemistry exam in 2 weeks and was hoping to get some help with questions I'm going through from previous years exams that were were given without answers and I'm stuck on.
( Read more... ) -
Gallium oxides
Dear сolleagues,
I had an unsolved problem. I have a gallium oxide, which is a mixture of three modifications: α-, β- and ε-Ga2O3.
The question: is how to get rid of the β-Ga2O3 in this mixture?
P.S. Option, described in Wikipedia (heating β-Ga2O3 at 65 kbar and 1100 C) is not considered, the pressure does not build.
Is there some other way? -
Chemical Equilibrium
Hey it's me again! I have a question about chemical equilibrium.
At temperatures near 400 C, the Kp value for the synthesis of ammonia is 2.5 x 10-4. At 400 C, what would be the approximate value of K for the following reaction?
3H2(g) + N2 (g)⇔ 2NH3 (g)
So, the equation for the partial pressure is Kp = Kc(RT)Δn
Δn-2-4= -2
To solve for Kc, it' ( Read more... ) -
Reaction Rates
Hey, I was wondering if anyone could help me with a problem regarding reaction rates
For the following reaction in a 4.00-L container, it was found that 1.00 x 10-4 mol of C4H8 reacted over a time period from 10:58 A.M. to 11:15 A.M.
C4H8 (g) --> 2C2H4 (g ( Read more... ) -
10% solution
HI ( Read more... ) -
(Untitled)
Does anyone know how to remove the acetate from this molecule, leaving a terminal olefin? It's not homework, I'm doing a past exam paper as revision and the final molecule has no OAc, but the first part of the question was about selective acetylation?
Thanks so much! :)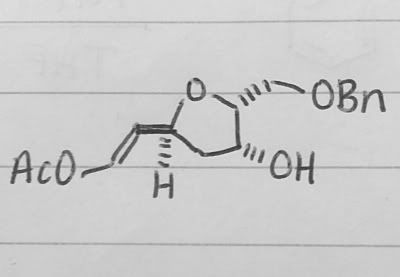
( Read more... ) -
Estimating Delta G at a given temperature?
In class a few days we are given this problem. I understand the first part because, but I have no idea how to estimate Delta G at 400 K. The answer is provided, but can anyone help inform me on how that answer was obtained?
Use a table of thermodynamic properties to determine
( Read more... )
